
The FDA issued a warning Wednesday to consumers not to purchase or use products marketed with variations of the names “Artri” or “Ortiga” due to potentially dangerous hidden active drug ingredients not listed on the product label. FDA urges consumers taking these products to immediately talk to their health care professional (e.g., doctor) to safely discontinue use of the product because suddenly stopping these drugs may be dangerous.
These products are promoted for treating arthritis, muscle pain, osteoporosis, bone cancer, and other conditions and are sold on various websites and in some retail stores.
FDA laboratory analyses revealed certain Artri and Ortiga products contain the undeclared drug ingredients:
- Dexamethasone (a corticosteroid) that can cause serious adverse events, including infections, increased blood glucose (sugar) levels, changes in blood pressure, damage to bones, psychiatric problems, and adrenal dysfunction;
- Diclofenac sodium (an anti-inflammatory drug) that can lead to adverse cardiovascular events, such as heart attack and stroke, or serious gastrointestinal damage, including bleeding, ulceration, and fatal tears of the stomach and intestines, or liver toxicity including liver failure that can cause the need for a liver transplant or death
- Methocarbamol (a muscle relaxant) that can cause sedation, dizziness, and low blood pressure.
These drug ingredients, which are not listed on the product label, can also interact with other drugs a consumer is taking.

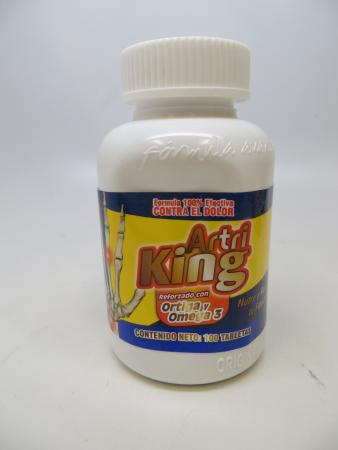
FDA has received adverse event reports, including of liver toxicity and death, associated with the use of Artri King products, since the agency issued its first warning about an Artri Ajo King product on January 5, 2022.
Suddenly stopping corticosteroids after long-term use or high doses can result in a serious withdrawal syndrome that includes fatigue, nausea, low blood pressure, low blood glucose levels, fever, dizziness, muscle and joint pain, and shortness of breath. These risks depend on several factors that a health care professional must assess. Medical intervention may be necessary.
Health care professionals should evaluate patients who have used Artri and Ortiga products for drug and disease interactions involving diclofenac, methocarbamol, and corticosteroids, and treat accordingly.
FDA has identified the following Artri and Ortiga products containing hidden drug ingredients:

- Artri Ajo King – a product promoted and sold on various websites, including www.amazon.com, and possibly in some retail stores.
- Artri King – a product promoted and sold on various websites, including www.amazon.com, www.latinfoodsmarket.com, and www.walmart.com and possibly in some retail stores.
- Ortiga Mas Ajo Rey – product promoted and sold on various websites, including www.amazon.com, and possibly in some retail stores.
- Ortiga Mas Ajo Rey Extra Forte – a product promoted and sold on various websites, including www.ebay.com, and possibly in some retail stores.
FDA analyses reflect only the undeclared ingredients discovered in one product from a specific lot, but ingredients may vary from product to product or from lot to lot. Products marketed as dietary supplements that are found to have hidden drug ingredients generally fail to comply with most current good manufacturing practices designed to ensure product quality and safety. Therefore, consumers should expect the manufacturing processes for Artri and Ortiga products are unreliable in providing consistent amounts of active ingredients or to prevent the introduction of unknown chemicals or other impurities.

FDA is investigating the distribution of these products in the United States and has advised certain companies not to sell or distribute these products. The agency may take additional enforcement steps that may include warning letters, seizure, injunction, or criminal charges.
Health care professionals and consumers should report adverse events or side effects related to the use of this product to FDA’s MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report online at www.fda.gov/medwatch/report.htm; or
- Download and complete the form, then submit it via fax at 1-800-FDA-0178.





