Jan. 12 Deadline For Rural Hospitals, Nursing Facilities To Apply For ARPA COVID-19 Funding
AUSTIN – The Texas Health and Human Services Commission is reminding rural hospitals and nursing facilities in Texas to apply for $128 million in federal American Rescue Plan Act funding to help pay for critical staffing needs during the COVID-19 pandemic. The deadline to apply is Wednesday, Jan. 12. “We’re encouraging health care providers who have worked so hard in responding to the COVID-19 pandemic to take advantage of this...
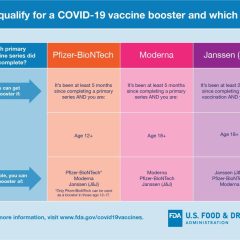
FDA Shortens Interval For Booster Dose of Moderna COVID-19 Vaccine to 5 Months
FDA News Release – Jan. 7, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Moderna COVID-19 Vaccine to shorten the time between the completion of a primary series of the vaccine and a booster dose to at least 5 months for individuals 18 years of age and older. “The country is in the middle of a wave of the highly contagious omicron variant, which spreads more rapidly than...
FDA Acts To Expand Use Of Pfizer COVID-19 Vaccine
FDA Jan. 3, 2022 News Release Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine to: Expand the use of a single booster dose to include use in individuals 12 through 15 years of age.Shorten the time between the completion of primary vaccination of the Pfizer-BioNTech COVID-19 Vaccine and a booster dose to at least five months.Allow for a third primary...
First Known Case Of COVID-19 Omicron Variant In Texas Identified In Harris County
DSHS News Release – 6:59 p.m. Monday, Dec. 6, 2021 The first known Texas case of the COVID-19 B.1.1.529 variant has been identified in a resident of Harris County. The adult female resident was recently diagnosed with COVID-19. Results of genetic sequencing this week showed that the infection was caused by the Omicron variant strain. The case is being investigated by Harris County Public Health and the Texas Department of State...
FDA Expands Eligibility For COVID-19 Vaccine Booster For All Individuals 18 Or Older
FDA News Release – November 19, 2021 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUA) for both the Moderna and Pfizer-BioNTech COVID-19 vaccines, authorizing use of a single booster dose for all individuals 18 years of age and older after completion of primary vaccination with any FDA-authorized or approved COVID-19 vaccine. The Centers for Disease Control and Prevention’s (CDC)...
DSHS: Texas Data Shows Unvaccinated People 20 Times More Likely To Die From COVID-19
DSHS Nov. 8, 2021 News Release A new study released by the Texas Department of State Health Services shows that during the month of September, Texans not vaccinated against COVID-19 were about 20 times more likely to suffer a COVID-19-associated death and 13 times more likely to test positive than people who were fully vaccinated. An analysis of data from the four-week period from Sept. 4 through Oct. 1 shows that vaccination had a...
FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine For Emergency Use In Children 5 Through 11 Years of Age
Friday, Oct. 29, 2021 USFDA New Release Today, the U.S. Food and Drug Administration authorized the emergency use of the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 to include children 5 through 11 years of age. The authorization was based on the FDA’s thorough and transparent evaluation of the data that included input from independent advisory committee experts who overwhelmingly voted in favor of making the...
FDA Takes Additional Actions On Use Of A Booster Dose For COVID-19 Vaccines
US FDA News Release The U.S. Food and Drug Administration Wednesday, Oct. 20, took action to expand the use of a booster dose for COVID-19 vaccines in eligible populations. The agency is amending the emergency use authorizations (EUA) for COVID-19 vaccines to allow for the use of a single booster dose as follows: The use of a single booster dose of the Moderna COVID-19 Vaccine that may be administered at least 6 months after...
Rep. Slaton Calls For Legislation To Nullify President’s COVID-19 Vaccine Mandates
AUSTIN, TEXAS – Representative Bryan Slaton of House District 2 released the following statementregarding Governor Abbott’s Executive Order GA-40, which prohibits any entity in the State from requiring COVID-19 vaccinations, and adds the issue to the Special Session call. “Governor Abbott’s order is a much needed, but temporary, relief from the tyranny of vaccine mandates that are becoming more common among...
Slaton Files 3 Bills To Ban COVID-19 Vaccine Mandates
News Release – Oct 7, 2021 AUSTIN, TEXAS – Texas House District 2 Representative Bryan Slaton Thursday announced the filing of three pieces of legislation: House Bills 33, HB 110 and HB 125, to protect Texans against unjust COVID-19 vaccine mandates. HB 33 would prohibit companies and hospitals in Texas from forcing COVID-19 vaccines on their employees. HB 110 would stop state and local governmental entities, including...